The mechanical properties of materials depend strongly upon microstructure and the development of microstructure of an alloy is related to the characteristics of its phase diagram. Therefore, the understanding of phase diagrams for alloy systems is very important. Phase diagram is very useful to represent the most stable relationships between phases in alloy systems. In addition, phase diagrams provide valuable information about melting, casting, crystallization, and other phenomena.
Before interpret and utilize phase diagram, it is necessary to understand component, system and phase. Components are pure metals and/or compounds of which an alloy is composed. For example, in a copper–zinc brass, the components are Cu and Zn. System may refer to a specific body of material under consideration (e.g., a ladle of molten steel). Or it may relate to the series of possible alloys consisting of the same components, but without regard to alloy composition (e.g., the iron–carbon system). Phase may be defined as a homogeneous portion of a system that has uniform physical and chemical characteristics. Every pure material is considered to be a phase; so also is every solid, liquid, and gaseous solution.
Unary System
Perhaps the simplest and easiest type of phase diagram to understand is that for a one-component system. In a one-component system, or unary system, however, the composition does not vary, but must always be unity. Therefore there are only two variables which can vary: pressure and temperature. Every possible combination of temperature and pressure can be readily represented by points on a two-dimensional diagram. Three phases (solid-, liquid, and vapor-phase) are found on this type of phase diagram.
Binary System
Another type of common phase diagram is that for two components or binary system. Binary phase diagrams represent the relationships between temperature and the compositions of phases at equilibrium at constant external pressure. Areas, or phase regions, are defined on these temperature-versus-composition plots within which either one or two phases exist. For an alloy of specified composition and at a known temperature, the phases present, their compositions, and relative amounts under equilibrium conditions may be determined.
Binary Systems without Solid Solution
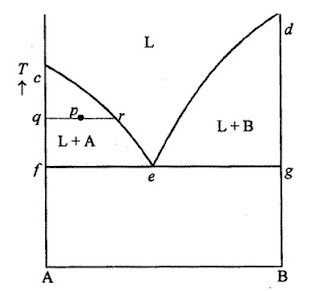
Consider a system of two components, A and B, which are completely soluble in one another in the liquid state, but completely insoluble in one another in the solid state. The melting point of a liquid is normally depressed if the liquid contains some other substance in solution.
Binary Systems with Total Solid Solubility (Binary Isomorphous Systems)
It is possible for solids to form a solution. Solid solution means that the solute component enters and becomes a part of the crystalline solvent, without altering its basic structure. Solid solutions with complete solid solubility, i.e., solid solubility over the entire range of the composition, are possible to form. For a metallic binary solution to exhibit a complete solid solubility, for instance, both metals must have the same type of crystal structure, nearly identical atomic radii and electronegativities, and similar valences because it must be possible to replace all the atoms of the initial solvent with solute atoms without causing a change in crystal structure. The copper–nickel system displays this behavior. The copper–nickel system is termed isomorphous because of this complete liquid and solid solubility of the two components. The phase diagram shapes are as shown below:
Three different phase regions, or fields, appear on the diagram, an alpha (α) field, a liquid (L) field, and a two-phase field. The liquid L is a homogeneous liquid solution composed of both A and B. The α-phase is a substitutional solid solution consisting of both A and B atoms. At temperatures below about A and B are mutually soluble in each other in the solid state for all compositions.
Binary Systems with Partial Solid Solubility (Binary Eutectic System)
In many cases, atom size, crystal structure or other factors restrict the ease with which solute atoms can be dissolved in the solvent in the solid state. Thus it is much more common to find that solids are partly soluble in one another rather than be either completely soluble or completely insoluble. The copper–silver system displays this behavior. The following is a binary system which shows partial solid solubility:
Three single-phase regions are found on the diagram: α, β, and liquid. The α-phase is a solid solution rich in A atom; it has B atom as the solute component. Otherwise in β-phase solid solution, A is the solute. Pure A and pure B are also considered to be α- and β-phases, respectively.
There are also three two-phase regions found for the system: The α+L, β+L and α+β. The α– and β-phase solid solutions coexist for all compositions and temperatures within the α+β phase field; the α+liquid and β++liquid phases also coexist in their respective phase regions.
References:
Callister, W.D. 2007. Materials science and engineering : an introduction 7ed
Lee, H.G. 2000. Chemical Thermodynamics for Metals And Materials
Before interpret and utilize phase diagram, it is necessary to understand component, system and phase. Components are pure metals and/or compounds of which an alloy is composed. For example, in a copper–zinc brass, the components are Cu and Zn. System may refer to a specific body of material under consideration (e.g., a ladle of molten steel). Or it may relate to the series of possible alloys consisting of the same components, but without regard to alloy composition (e.g., the iron–carbon system). Phase may be defined as a homogeneous portion of a system that has uniform physical and chemical characteristics. Every pure material is considered to be a phase; so also is every solid, liquid, and gaseous solution.
Unary System
Perhaps the simplest and easiest type of phase diagram to understand is that for a one-component system. In a one-component system, or unary system, however, the composition does not vary, but must always be unity. Therefore there are only two variables which can vary: pressure and temperature. Every possible combination of temperature and pressure can be readily represented by points on a two-dimensional diagram. Three phases (solid-, liquid, and vapor-phase) are found on this type of phase diagram.
(www.teachersparadise.com)
Binary System
Another type of common phase diagram is that for two components or binary system. Binary phase diagrams represent the relationships between temperature and the compositions of phases at equilibrium at constant external pressure. Areas, or phase regions, are defined on these temperature-versus-composition plots within which either one or two phases exist. For an alloy of specified composition and at a known temperature, the phases present, their compositions, and relative amounts under equilibrium conditions may be determined.
Binary Systems without Solid Solution
Consider a system of two components, A and B, which are completely soluble in one another in the liquid state, but completely insoluble in one another in the solid state. The melting point of a liquid is normally depressed if the liquid contains some other substance in solution.
Binary Systems with Total Solid Solubility (Binary Isomorphous Systems)
It is possible for solids to form a solution. Solid solution means that the solute component enters and becomes a part of the crystalline solvent, without altering its basic structure. Solid solutions with complete solid solubility, i.e., solid solubility over the entire range of the composition, are possible to form. For a metallic binary solution to exhibit a complete solid solubility, for instance, both metals must have the same type of crystal structure, nearly identical atomic radii and electronegativities, and similar valences because it must be possible to replace all the atoms of the initial solvent with solute atoms without causing a change in crystal structure. The copper–nickel system displays this behavior. The copper–nickel system is termed isomorphous because of this complete liquid and solid solubility of the two components. The phase diagram shapes are as shown below:
(source: www.soton.ac.uk)
Three different phase regions, or fields, appear on the diagram, an alpha (α) field, a liquid (L) field, and a two-phase field. The liquid L is a homogeneous liquid solution composed of both A and B. The α-phase is a substitutional solid solution consisting of both A and B atoms. At temperatures below about A and B are mutually soluble in each other in the solid state for all compositions.
Binary Systems with Partial Solid Solubility (Binary Eutectic System)
In many cases, atom size, crystal structure or other factors restrict the ease with which solute atoms can be dissolved in the solvent in the solid state. Thus it is much more common to find that solids are partly soluble in one another rather than be either completely soluble or completely insoluble. The copper–silver system displays this behavior. The following is a binary system which shows partial solid solubility:
(source: www.soton.ac.uk)
Three single-phase regions are found on the diagram: α, β, and liquid. The α-phase is a solid solution rich in A atom; it has B atom as the solute component. Otherwise in β-phase solid solution, A is the solute. Pure A and pure B are also considered to be α- and β-phases, respectively.
There are also three two-phase regions found for the system: The α+L, β+L and α+β. The α– and β-phase solid solutions coexist for all compositions and temperatures within the α+β phase field; the α+liquid and β++liquid phases also coexist in their respective phase regions.
References:
Callister, W.D. 2007. Materials science and engineering : an introduction 7ed
Lee, H.G. 2000. Chemical Thermodynamics for Metals And Materials